Access to an essential treatment known as CAR T-cell therapy, or Carvykti, which is used to treat adults with multiple myeloma—an incurable form of blood cancer—is currently facing delays in Canada, according to health advocates. This one-time treatment has shown positive results in the United States, offering patients a chance for extended life spans. However, negotiations for national reimbursement between Johnson & Johnson, the drug manufacturer, and the Pan-Canadian Pharmaceutical Alliance (PCPA) ended in September 2025 without a resolution.
The termination of these discussions implies that thousands of Canadians suffering from multiple myeloma, who would be eligible for this treatment, may have to pay the exorbitant price of $632,455 out of pocket. "At some point, they just couldn’t agree on the price that they were willing to pay or offer the province," stated Martine Elias, CEO of Myeloma Canada. "I think there is an opportunity to go back to the table and restart the negotiation because it’s in the interest of the patients."
A Mother's Anxiety
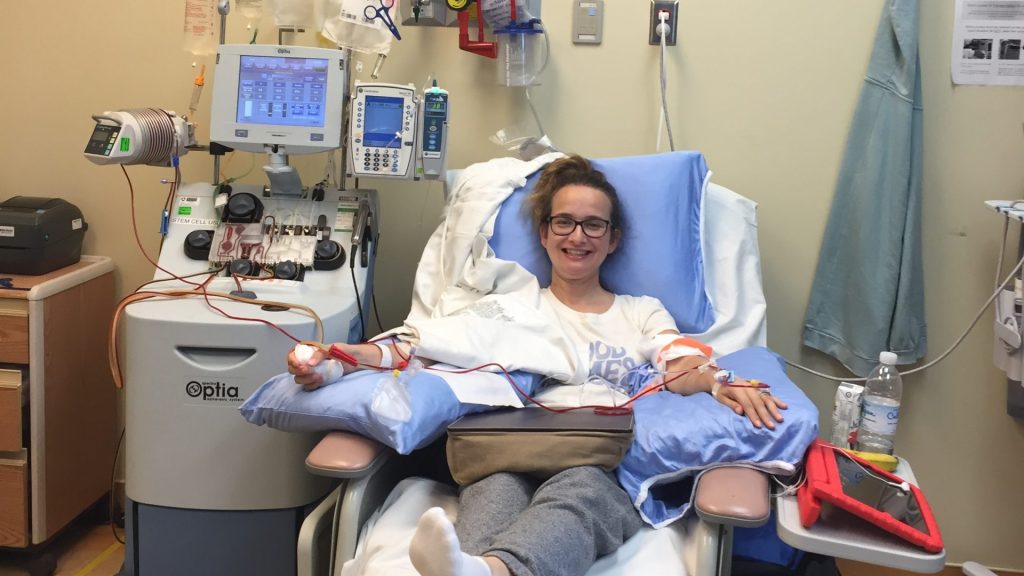
Tanya Zigomanis, a mother of two, was diagnosed with multiple myeloma in 2019, at the age of 37. Since her diagnosis, she has undergone several rounds of chemotherapy and a stem cell transplant. After experiencing a relapse in March, she has also required biweekly IV chemotherapy. Zigomanis expresses concern that she may one day need Carvykti as an additional treatment option, yet she is apprehensive about the financial burden it entails.
"I’m scared I’m going to ask family and friends to put together funds for me, I’m scared I’m going to have to sell my house to literally go to the U.S. to have CAR T-cell therapy there," she explained. "I know a few friends who have done so." Zigomanis is just one among thousands of Canadians who may face such tough decisions if funding for this therapy is not approved, with nearly 4,000 new cases of multiple myeloma diagnosed in Canada each year.
"This medication would mean many drug-free years without going into hospitals for treatment and being able to live an everyday life, which most people can’t do right now," added Zigomanis.
Negotiation Stalemate
CityNews reached out to both Johnson & Johnson and the PCPA for comments on the situation. A spokesperson from Johnson & Johnson indicated that the PCPA was seeking a single price applicable across all provinces, aligning with its goals to achieve savings for public pharmacare plans. However, in Johnson & Johnson’s perspective, this demand has been obstructing provinces that are ready and willing to make Carvykti available.
In contrast, a representative from the PCPA stated that during negotiations, there was a significant discrepancy between the price suggested by Johnson & Johnson and the perceived value of the drug. The exact figures discussed during these confidential negotiations remain undisclosed. Although talks have concluded, the PCPA mentioned that it would be open to rekindling discussions should the drug manufacturer submit a revised proposal.
When inquired whether Johnson & Johnson would resubmit an offer, a company spokesperson refrained from providing a definitive answer, stating that the company will "continue to explore every option that enables the timely availability of this therapy."
In the interim, thousands of Canadians grappling with multiple myeloma continue to await clarity regarding access to this critical treatment while contending with a disease that does not wait.